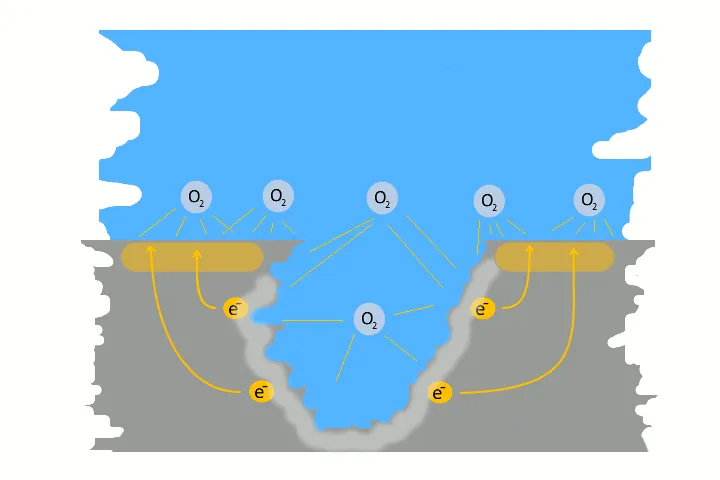
If corrosion occurs in metal pipes, the pipes might start leaking over time. In the vernacular this is also called „pitting corrosion“, although this is not really correct. It is the case, if a pit builds on the inside of the pipe or machine.
Pitting corrosion is usually the result of galvanic corrosion. This occurs when two different metals and water come together as electrolytes. This creates charges – like in a battery, electrons flow. The result is a small pit. The electrons flow from the base metal to the noble metal and produce the pitting.
Therefore it is good craftsmanship practice, if in a mixed installation, a piece of brass, is put in between the iron and the copper.
Pitting step by step or page after page

Very often pitting starts, if impurities such as iron particles, enter into a water system. If the iron particle meet a nobler metal, as copper alloy or stainless steel. These iron particles form many small galvanic elements in small areas. The corrosion starts immediately and produce pitting. The material of the pipe is not essential, whether copper, aluminium alloy, stainless steel or iron. The decisive factor is the potential difference, between the pipe material and the foreign particle.
Here in the picture on the left we show a detailed technical version of how corrosion and pitting occurs. Explanations to each picture and partly graphically animated.
Depending on the water and the quality of the pipe, this process takes place more quickly in areas, where the surface have already been attacked, compared to a surface whose still intact. Over time, the pitting build a pit and eats its way through the pipe. This becomes leaky and a hole develops. This piece of pipe with the pinhole leak must either be repaired or replaced.
The damage can be quite high. Repairing the pipe is not the problem. It’s the damage the leaking water can cause. The escaping water that is distributed in walls and floors. Frequently to localize the spot in the pipe, which has the pitting corrosion, is extremely laborious and complex.
The classical ways of corrosion products, such as corrosion inhibitors, cathodic protection are often not efficient. The chemicals dont reach the corrosion pit.
Preventive against pitting corrosion
The Merus Ring can be used preventively. To reduce the likelihood of pitting in the first place. Or in the best case to stop an existing acute pitting corrosion. Of course, Merus can only influence corrosion or pitting that occurs from the inside out. Merus is not suitable for preventing corrosion on the outside of the pipe.
We are not one hundred percent able to prevent pitting corrosion. The reason for this is that strong chemical forces act when different elements react. However, experience in projects implemented by Merus shows that the frequency of pipe damage has decreased by more than 90%. This is achieved, because Merus Rings are forming a protective oxide film on the metal surface. Known also as magnetite. This magnetite on the inner surface in contact with the water, act as some sort of passivation. this oxide film increase the corrosion resistance.
In a particularly drastic case at a customer with around 100 residential properties, pipe damage had to be repaired every three to four months beforehand. After the installation of Merus 8 years ago, two more cases have occurred since then.
Another case is the Tour Odeon in Monaco. There rust particles have appeared in the stainless steel pipes. These caused corrosion on the horizontal pipes, first on the surface and then to the pitting and finally lead to a considerable financial loss. What causes the problem, i.e. where the rust particles come, has not yet been clearly clarified.


Comments are closed