RE300 is a rare earth-based coagulant for use in water treatment. It is mainly composed of the ionic forms of Cerium (Ce) but can contain other rare earths such as Lanthanum (La), Neodymium (Nd), and Praseodymium (Pr) among others. RE300 is stable in a solution with a pH between 3 and 4. One of the many benefits of RE300 it is significantly less acidic than other metal salts used in water treatment. This document will focus on reasons why a less acidic chemical can be a benefit in water treatment. The following definitions are given to help clear up some confusion on the subjects of acid/base chemistry and alkalinity.
· pH – the pH scale is a measure of acid and base concentration. It ranges from 1 to 14 with 7 considered neutral. The pH scale is logarithmic which means that every integer change results in a 10x higher acid or base concentration.
Example; pH 6 is 10 times more acidic than pH 7 and pH 4 is 1,000 times more acidic than 7.
· Alkalinity – Alkalinity is the ability of a solution to resist pH changes when an acid is added. The acid molecules react with the alkalinity which results in the acid molecules being neutralized, therefor when adding acid to a solution with alkalinity, the pH stays constant until the alkalinity is consumed. This is the reason adding acidic water treatment chemicals consumes alkalinity. In municipal and industrial wastewater there are many factors which contribute to alkalinity. Factors which contribute to alkalinity include the type of dissolved inorganic and organic compounds present in the water, the amount of suspended organic matter in the water, and the amount of bicarbonate in the water.
· Acid – An acid is anything that will donate a proton (a proton is the same thing as a hydrogen ion H+) in solution. A lower pH acid is simply a higher concentration of protons in solution. A pH of 1 is the lowest number on the scale and therefor the most acidic measurement on the pH scale.
· Base – A base is anything that will release a hydronium ion (OH-) in solution, or anything that will consume an acid H+. A pH of 14 is the most basic measurement on the scale. Acids and bases are linked because when you combine them in equal amounts the H+ bonds with the OH- to create water H2O. Bases are used in water treatment to adjust the pH if the water becomes acidic.
NOTE: bases are often referred to as caustic or alkaline solutions. It is important to note that an alkaline solution such as caustic soda does not add alkalinity to the solution, instead if directly modifies the pH. By contrast, a solution can be neutral and have a high alkalinity. A solution with high alkalinity would consume added acid without changing pH until the alkalinity is gone, but any additional acid will then affect the pH.
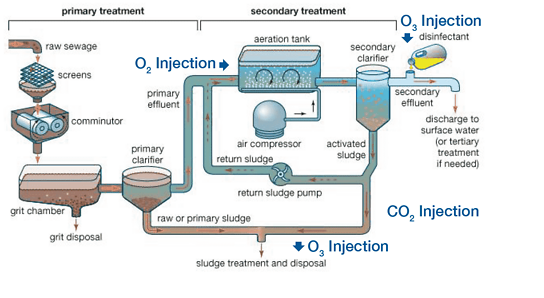
In Wastewater
The bacteria and other organisms which play an active role in wastewater treatment are most effective at a neutral to slightly alkaline pH of 7 to 8. To maintain these optimal pH conditions for biological activity there must be sufficient alkalinity present in the wastewater to neutralize acids generated by the active biomass during waste treatment especially nitrification. This ability to maintain the proper pH in the wastewater as it undergoes treatment is the reason why alkalinity is so important to the wastewater process. If all alkalinity in the wastewater process is consumed, an alkaline solution such as caustic soda or magnesium hydroxide can be added to maintain the system pH between 7-8 as the denitrifying bacteria generate acid but this adds cost and complexity to the system.
RE300 pH Benefit
Due to the more acidic pH of iron/aluminum there is also the potential of lowering the entire water treatment system pH when using these coagulants. Because of the logarithmic nature of the pH scale, ferric chloride is 100,000 times more acidic than neutral water. Consequently, even a small addition of ferric chloride can reduce the system pH enough that a base such as caustic soda would be needed to raise the system pH back to neutral to meet the discharge allowable pH. Once again RE300 is 100 times less acidic than ferric chloride and typically requires a much lower dose. This results in less change to the system pH and likely no need to adjust the pH to stay in the neutral range for effluent discharge regulations.