Nitrogen is an essential element required by all organisms. In animals, nitrogen is a component of crucial organic molecules, such as proteins and DNA, and it constitutes 1 to 3% dry weight of cells. Our atmosphere contains 78% by volume of nitrogen, yet it is not a common element on Earth. Although nitrogen is an essential ingredient for plant growth, it is chemically very inactive, and it must be fixed before the vast majority of the biomass can incorporate it. Special nitrogen-fixing bacteria found in soil and water fix nitrogen; thus, microorganisms play a major role in nitrogen cycling in the environment. These microorganisms (bacteria) have the ability to take nitrogen gas from the air and convert it to nitrate via a process known as nitrogen fixation. Some of these bacteria occur as free-living organisms in the soil. Others live in a symbiotic relationship with plants. A symbiotic relationship is a close relationship between two organisms of different species where both partners benefit from the association. An example of a symbiotic relationship related to nitrogen can be seen, for example, in the roots of
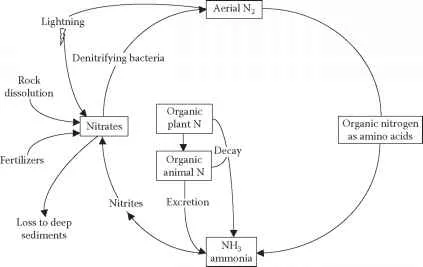
Figure 8.18 Nitrogen cycle.
peas. These roots have small swellings along their length. These contain millions of symbiotic bacteria that have the ability to take nitrogen gas from the atmosphere and convert it to nitrates that can be used by the plant. The plant is then plowed back into the soil after the growing season to improve the nitrogen content.
Price (1984) described the nitrogen cycle as an example “of a largely complete chemical cycle in ecosystems with little leaching out of the system.” Simply, the nitrogen cycle provides various bridges between the atmospheric reservoirs and the biological communities (see Figure 8.18).
Atmospheric nitrogen is fixed by either natural or industrial means; for example, nitrogen is fixed by lightning or by soil bacteria that convert it to ammonia, then to nitrite, and finally to nitrates, which plants can use. Nitrifying bacteria make nitrogen from animal wastes. Denitrifying bacteria convert nitrates back to nitrogen and release it as nitrogen gas.
The logical question now is “What does all of this have to do with water?” The best way to answer this question is to ask another question. Have you ever dived into a slow-moving stream and had the noxious misfortune to surface right in the middle of an algae bloom? When this happens to you, the first thought that runs through your mind is, “Where is my nose plug?” Why? Because of the horrendous stench, disablement of the olfactory sense is a necessity.
If too much nitrate, for example, enters the water supply as runoff from fertilizers, it produces an overabundance of algae known as an algae bloom. If this runoff from fertilizer gets into a body of water, algae may grow so profusely that they form a blanket over the surface. This usually happens in summer, when the light levels and warm temperatures favor rapid growth.
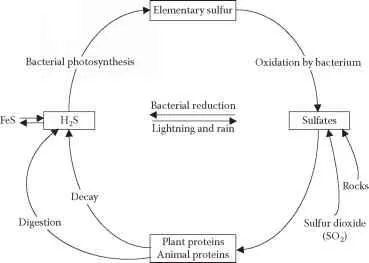
Figure 8.19 Sulfur cycle.
In the voluminous and authoritative text Wastewater Engineering: Treatment, Disposal, & Reuse (Metcalf & Eddy, 2003), it is noted that nitrogen is found in wastewater in the form of urea. During wastewater treatment, the urea is transformed into ammonia nitrogen. Because ammonia exerts a BOD and chlorine demand, high quantities of ammonia in wastewater effluents are undesirable. The process of nitrification is utilized to convert ammonia to nitrates. Nitrification is a biological process that involves the addition of oxygen to the wastewater. If further treatment is necessary, another biological process called denitri-fication is used. In this process, nitrate is converted into nitrogen gas, which is lost to the atmosphere, as can be seen in Figure 8.18. From the wastewater operator’s point of view, nitrogen and phosphorus are both considered limiting factors for productivity. Phosphorus discharged into streams contributes to pollution. Of the two, nitrogen is more difficult to control but is found in smaller quantities in wastewater.
Comments are closed