Phosphorous and potash are the essential elements for plant growth. The importance of phosphate and potash in soil in agriculture has long been recognized. A predominantly agricultural country like India needs a vast amount of fertilizers. Phosphates are regarded as one of the most important fertilizer minerals used by man. Nearly 90% of the total world phosphate output is utilized for the manufacture of a number of fertilizers such as superphosphate, triple-superphosphate, di-ammonium-phosphate and mono-ammonium phosphate, which are essential for agro-industries all over the globe.Rock phosphate also referred to as phosphorites, are sedimentary deposits with high phosphorus (P) concentrations. These rocks are one of the primary sources for P which in turn is a critical and nonrenewable element for fertilizer production upon which global fertility depends. Phosphorite is a non-detrital sedimentary rock, which contains 18–20% phosphate-bearing minerals [1-11].Typical phosphorite beds contain about 30% P2O5 and constitute the primary source of raw materials for most of the world’s production of phosphatic fertilizers. Alteration of phosphorites tends to leach carbonates and increase the percentage of P2O5. The Phosphoria Formation contains about 34% P2O5near the surface as compared to depth where it is about 28% [12]. Phosphorite deposits generally occur in three principal types: (i) regionally extensive crystalline nodular and bedded deposits; (ii) localized concentrations of phosphate-rich clastic deposits (bone beds); and (iii) guano deposits. Broadly, phosphates can be grouped as: (i) primary phosphates that have crystallized from a liquid; (ii) secondary phosphates formed by the alteration of primary phosphates; and (iii) fine-grained rock phosphates formed at low temperatures from phosphorus-bearing organic material primarily underwater.
In the study area, phosphorites are found to occur as lenticular and detached bodies throughout the Formation of the Bijawar Group. Individual bodies range from a few meters to about 4 km in length and width varies from thin bands to about 125 m with P2O5 concentration ranging from 10 to 20%. Estimated reserves of the Precambrian phosphorites of Lalitpur area are 5 million tons with an average grade of 25% based on detailed studies of two prominent lensoid bodies of phosphorites [9-11, 13, 14]. The phosphorites of the Sonrai basin of Lalitpur district in Uttar Pradesh was discovered in (1977) [13]. District of Lalitpur (Latitude 24° 11’N and 25° 13’N and Longitude 78° 11’E and 79° 0’E is surrounded by the adjacent district Jhansi in the north, Sagar (M.P.) in the south, Tikamgarh and Chhatarpur districts in the East and Shivpuri and Guna districts (M.P.) in the West. The western boundary of the district runs along river Betwa for about 96 kms. The southwestern boundary is formed by Narain Nandi, the northeastern by river Jamni for about 65 km and the southeastern boundary for about 40 kms by Dhasan Nadi. The Sonrai basin in Lalitpur district is located at the extreme south-west corner of Uttar Pradesh. The basin is 28 km long and 5 km wide and shows an east–west extension. The study area follows the regional east–west strike and forms a westward plunging syncline on a regional scale with closure on the eastern side. They are faulted against the northern crystalline rocks and the Berwar formations to the south. East and west are covered by the flat-lying Vindhyan conglomerate and sandstone [15, 16]. These deposits have been investigated by many earlier workers from the point of view of their geological and economic importance. However a detailed study of the geochemical characteristics of these rocks has not been done so far. In the present study, new geochemical data of advanced nature on Lalitpur phosphorites are utilized to deal with the major, trace, and rare earth element geochemistry in order to determine their abundance, distribution pattern, and interelement relationship of phosphorites ultimately to interpret their impact on phosphatic mineralization. Therefore, the present investigation may be considered as a first serious attempt to study the geochemistry of the Lalitpur phosphorite deposits in order to present a conceptual genetic model not only for these deposits but also to apply the same in areas of similar geologic and environmental setting.
Aim and objective
The present investigation has been, thus, undertaken with a view:
· To evaluate the governing factors involved during the process of phosphorite formation
· To understand the chemical and biological processes in the phosphorite deposition
· To study the mineralogy and chemistry of the various rock units in order to understand the genesis of the various minerals present in the phosphorites and to assess the quality of the deposit
Geological background
The Paleoproterozoic meta-sedimentary rocks of Bijawar Group are overlain by the Archean Bundelkhand Basement Complex and underlain by the Vindhyan Supergroup. The Sonrai rift basin preserves volcano-sedimentary units of Late Archaean to early Proterozoic age [17]. The Precambrian phosphorites of Bijawar Group of rocks show characteristics of an epicontinental sea with restricted and very shallow marine environment of formation along some shoals which existed during the iron-rich Precambrian times [18]. The Bijawar basin in Lalitpur district is located at the extreme southwest corner of Uttar Pradesh (Fig. 1) [6, 7, 9-11,15,16]. The basin is 28 km long and 5 km wide and shows an east–west extension.
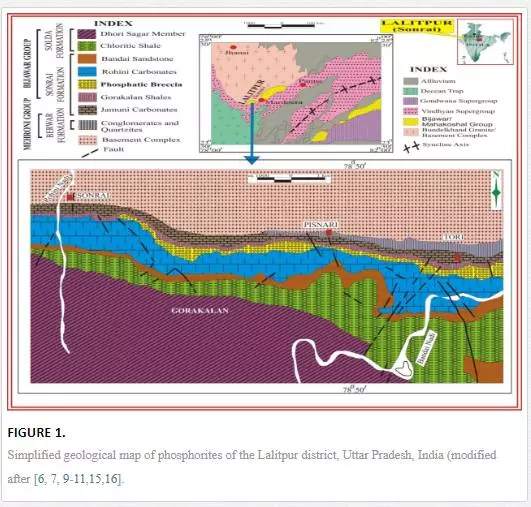
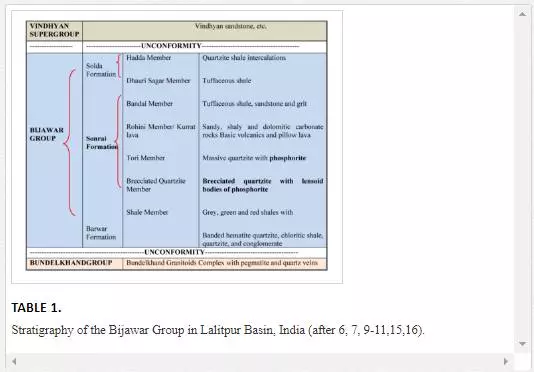
The lithostratigraphy of the area [19] is given in Table 1. The exposed lithostratographic units of the Bijawar Group in the Lalitpur district are divided into three formations: Berwar, Sonrai, and Solda. The Berwar Formation at the base contains banded hematite–quartzite, chloritic shale, quartzite, and pebbly conglomerate. The overlying Sonrai Formation is divided into four members which in ascending order are:
(a) Shale Member consisting of dark-grey, olive-grey, reddish and black shales with carbonate and phosphorite bands;
(b) Brecciated Quartzite Member comprising brecciated quartzite and lensoid bodies of phosphorites with quartzite and phosphatic matrix cementing angular to subangular fragments of quartzite;
(c) Tori Member with ferruginous massive quartzite and massive botryoidal and brecciated phosphorites and
(d) Rohini Member with its coeval Kurrat Lava Member.
The Rohini Member consists of thinly bedded, light-grey shaly dolomitic and pink sandy carbonate rocks. The uppermost Solda Formation is divided into three members:
(a) the Bandai Member at the base consisting of sandstone and grits which are calcareous at places;
(b) the overlying Dhauri Sagar Member with tuffaceous shales and
(c) the uppermost Hadda Member comprising quartzite and shale intercalations.
Megascopic study reveals that the brecciated phosphorite is reddish brown in color and fine to medium grained with angular fragments of chert and quartz embedded in a groundmass of iron oxides and secondary silica intercalated with minor veins of chert and iron oxides. The rock is hard, compact, bedded, and ferruginous but nonlaminated. These brecciated phosphorites are gradually merged into the host rocks. Their structural characteristics are very similar to those of the associated rocks. They have undergone folding like other litho units in the area. As such they appear to be intraformational sedimentary breccia only. The rocks consist mainly of clasts of subangular to subrounded cherty materials, quartz (which may be derived from quartz-veins/reefs), quartzites, schistose rock fragments, and other host rocks of the Bijawar Group. The intergranular spaces of these brecciated phosphorites are filled with secondary silica, ironoxides, and calcareous material in the form of matrix, which varies from ferruginous mudstone to a ferruginous cherty mass. The brecciated phosphorites generally contains fragments of apatite and other phosphatic minerals embedded in dark-brown cherty matrix or sometimes ferruginous. Such brecciated phosphorite deposits have also been reported in metasediments of Udaipur area, Rajasthan [20] and Bijawar rocks of Sagar–Chhatarpur districts of Madhya Pradesh. Brecciated rocks rich in apatite and magnetite have also been reported from Singhbhum thrust belt areas of Jharkhand and phosphatic breccia in Proterozoic metasedimentary sequence of Betul district of Madhya Pradesh [21]. The phosphorite horizon in the Lalitpur area is associated with pink to white brecciated massive quartzite, shale, dolomite, and limestone of the basal unit. The phosphorites occur in the form of stratified lensoid bodies and appear to be of sedimentary residual type. These extend in length from 50 to 250 m and range from 5 to 35 m in thickness (Fig. 2a,b,c,d) [9, 11].

Analytical methods
With the help of geological maps and topographic sheets (54L/15, 54L/11, 54L/16) of the area sampling was carried out and 87 fresh unweathered representative samples (6″x 4″) were collected from the field at regular intervals. The samples were selected for chemical analysis. For preparation of solution, 50 gms of each sample was taken for crushing and powdering. The samples were crushed in a steel mortar to coarser fractions free from secondary association then they were handpicked and dried in the oven at 100ºC to eliminate the hygroscopic moisture. The coarse material was further crushed in porcelain mortar till it became medium to fine grained. Finally the material was powdered in centrifugal ball mill and sieved by standard sieve of -200 mesh. The powder of each sample was packed in bottles and numbered properly for analysis.The major element geochemical analysis was carried out at National Geophysical Research Institute (NGRI), Hyderabad. The major elements were determined from pressed pellets, which were prepared by using collapsible aluminum cups [22]. These cups are filled with boric acid and about 1 gm of the finely powder rock sample was put on the top of the boric acid and pressed under a hydraulic press at 20 tons pressure to get a pellet. The sample pellets were analyzed using a Philips MagiX PRO model PW–2440 wavelength dispersive X-ray fluorescence spectrometer (XRF) coupled with automatic sample changer PW 2540.
Trace and rare earth elements (REE) were determined by inductively coupled plasma-mass spectrometry (ICP-MS) technique using Perkin-Elmer, SCIEX, Model ELAN DRC-II system at National Geophysical Research Institute (NGRI), Hyderabad. Analytical solutions of the samples for geochemical analysis were prepared by open acid digestion method [25]. 50 mg (0.0500g) powder of rock sample was taken in a clean dried PTFE Teflon beaker. Each sample was moistened with a few drops of ultrapure water. Then, 10 ml of an acid mixture (containing 7:3:1 HF: HNO3: HClO4) was added to each sample. Samples were swirled until completely moist. The beakers were then covered with lids and kept overnight for digestion after adding 1 ml of 5 μg ml-1 Rh solution (to act as an internal standard). The following day, the beakers were heated on a hot plate at ~200°C for about 1 h and the lids were removed and the contents were evaporated to incipient dryness until a crystalline paste was obtained. The remaining residues were then dissolved in 10 ml of 1:1 HNO3 and kept on a hot plate for 10 min with gentle heat (70°C) to dissolve all suspended particles. Finally, the volume was made up to 250 ml with double distilled water (purified water) (18 MΩ) and stored in polythene bottles. This solution, stored in polythene bottles, was used for the determination of trace and REE elements by inductively coupled plasma–mass spectrometry techniques (ICP-MS). The instrumental and data acquisition parameters are same as given by [23]. The precision of ICP-MS data is better than ±6% RSD for all the trace elements and was obtained in all cases with comparable accuracy. All the available data were standardized against the international reference rock standards NIST-120C for phosphorites. The data of major and trace element analysis are given in Figs. 3 and 4.
Results and discussions
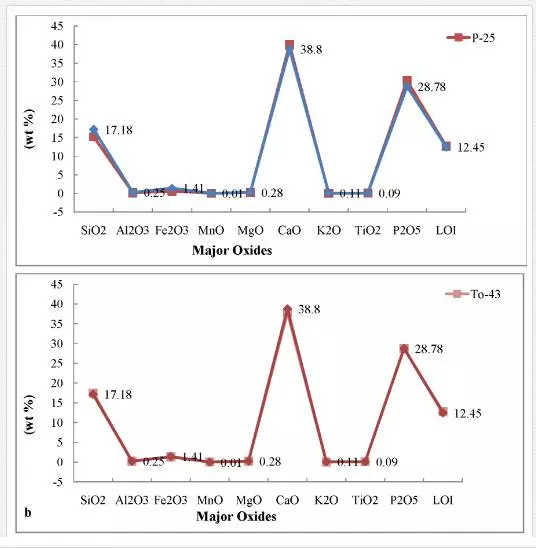
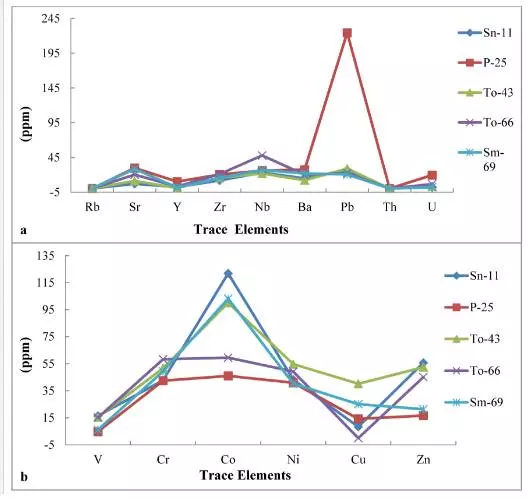
The abundance and concentration trend of major, trace and rare earth elements in the phosphorites of the Sonrai basin of Lalitpur district are presented in Figs. 3 and 4. The concentration trends of certain major oxides indicate that the phosphorites are more enriched in CaO, P2O5, and SiO2 than in Al2O3, Fe2O3, TiO2, Na2O, and K2O. The concentration trends of trace elements reveal that the phosphorites are moderately enriched in Co, Zn, Zr, Pb, and U than in Sc, Ba, V, Cr, Ni, Rb, Sr, Y, and Th.
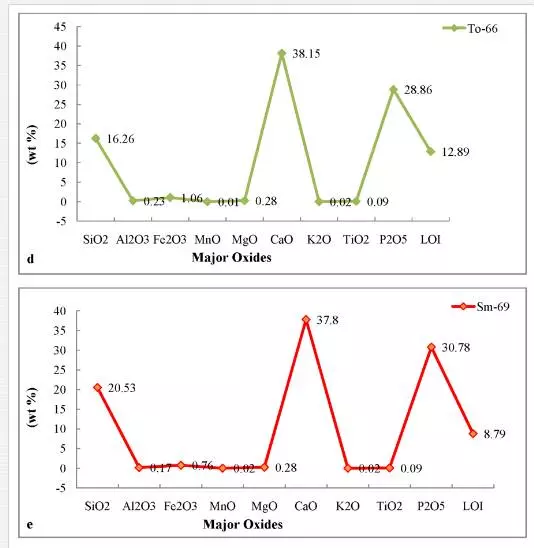
Phosphates are known to be deposited in a wide range of geological environments.
(1) Normally the phosphates are deposited in very shallow, near-shore, marine or low-energy environments. The possible environments are supratidal zones, littoral or intertidal zones, and estuarine zones.
(2) areas of oceanic upwelling cause the formation of phosphates because the constant stream of phosphate brought from the large, deep-ocean reservoir giving continuous growth of organisms.
(3)The genesis or origin of phosphorites is a controversial problem.
The complexity of the processes involved in the formation of marine phosphorite deposits has been the subject of study by a large number of investigators for a fairly long time. Several divergent views regarding the deposition and accumulation of phosphorites have earlier been put forward. The origin of phosphorites is not well understood and over the years there have been several hypotheses for their origin that include biological, chemical, and volcano-sedimentary [24, 25]. Many phosphorite deposits in the ocean occur in areas of upwelling, where phosphate, regenerated from the recycling of organic matter in the deep ocean is brought to coastal areas and triggers blooms of primary productivity such as along the coasts of Namibia[26] and Peru–Chile [27]. Nonupwelling areas such as estuaries and semi-restricted embayments can also be host to phosphate deposits or authigenic carbonate fluorapatite[26]. During early diagenesis, phosphorus may be released from organic matter and iron oxides under anoxic conditions, may play a vital role in phosphogenesis.
The geochemistry of phosphorites and their constituent mineral apatite have been widely studied owing to their economic importance and the potential utility of their geochemistry to estimate paleomarine chemistry. The variation in major and trace elements’ abundance is attributed to a change in environmental condition of deposition. The mobilizations of ferrous ions and subsequent precipitation as ferric oxides and oxyhydroxides have clearly redistributed iron in these phosphorites [28-32]. The same interpretation has been given by [33]for Ogun phosphate rocks.
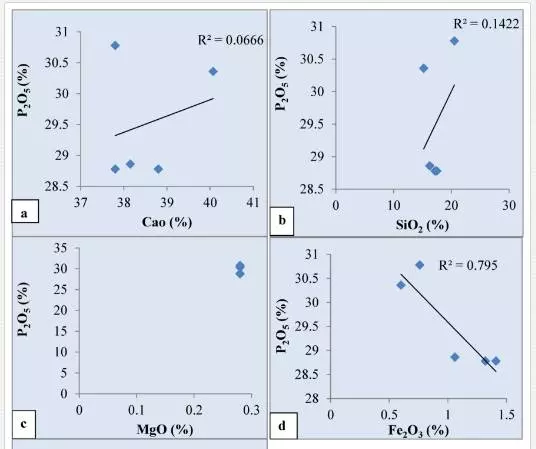
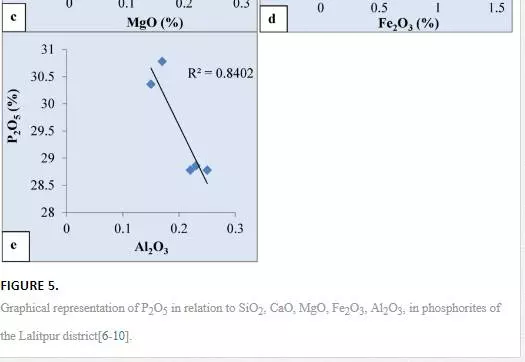
Higher values of P2O5 and CaO in the phosphorites of the area (Figs. 3, 4) indicate more concentration of apatite constituent. This corroborates to a greater extent to the Ogun phosphate rocks of Nigeria (34). Positive correlation of P2O5 and CaO (Fig. 5a) might be the result of diagenetic phosphatization in which CO2 might be removed by PO4 before the precipitation of phosphorite during high oxidizing conditions of the basinal water which gave rise to carbonate fluorapatite as an end product [6, 8].This relationship also indicates the diagenetic origin of phosphorites during highly oxidizing and alkaline basinal sea water[28, 35]. The positive correlation of P2O5 with SiO2(Fig. 5b) may be due to the presence of detrital minerals like quartz, feldspars, micas and clay bearing minerals. The positive relationship also suggests that much of SiO2 content is related to apatite. An inverse relationship between MgO and P2O5(Fig. 5c) in phosphorites indicates a continuous replacement of MgO by P2O5during diagenesis. Poor correlation between MgO and P2O5 in phosphorites of the area indicates that Mg might not have been incorporated in the apatite structure.The strong negative relationship between P2O5 with Fe2O3(Fig. 5d) in phosphorites may be due to leaching and/mild weathering of iron from the ores and reprecipitation along with P2O5 in the pore spaces, cavities/voids, veins, etc., in highly oxidizing marine environment of the Bijawar basin. This relationship between the constituents indirectly indicates the alkaline nature of basinal waters. Like iron, aluminum also decreases with the increase in P2O5 concentration (Fig. 5e). It may also be due to ionic substitution of Ca+2 by Al+3 in the carbonate fluorapatite phase due to weathering of the ores.The presence of low content of Al2O3 in the samples of phosphorites of the study area indicated the arid to humid climatic conditions. The low concentration of alumina may be due to minor occurrences of clay minerals and major content of ferruginous minerals like hematite, ferric-oxides, etc that constitute the cementing material of the phosphatic rocks. The presence of low Al2O3 in phosphorites indicates the humid to low temperature climatic conditions at the time of precipitation and phosphatization of these sediments in the area.
The term trace element is usually taken to mean those elements present in rocks in concentrations of less than a few thousands parts per million (ppm). Chemical elements like uranium (U), nickel (Ni), cobalt (Co), chromium (Cr), vanadium (V), copper (Cu), lead (Pb), gallium (Ga), etc., the content of which in rocks is rather appreciable and which form many minerals of their own, are commonly known as “trace elements”. In recent years, the chemical composition of rocks, more particularly the distribution of trace elements in sediments has been used for distinguishing and identifying precisely their depositional environment. Some of the earlier workers, particularly in the earlier years of this century, have emphasized the distribution and significance of trace elements in geological materials.Ba, Cu, Ni, and Zn are depleted in Lalitpur phosphorites in comparison to the average shale and phosphorite of Quaternary off southeast coast phosphate stromatolites formed under the influence of upwelling. This indicates that planktonic organic matter may not be the prime source of these elements in the Lalitpur phosphorite deposits. Since the sediments hosting phosphorites of Lalitpur were formed in shallow marine conditions, some of these elements may have recycled back to the overlying water column during sediment diagenesis in oxygenated environments. Pb, Zn, and Mo are below the detection limit in Lalitpur phosphorites suggesting that strongly reducing conditions were not prevailing at the time of phosphorite formation.
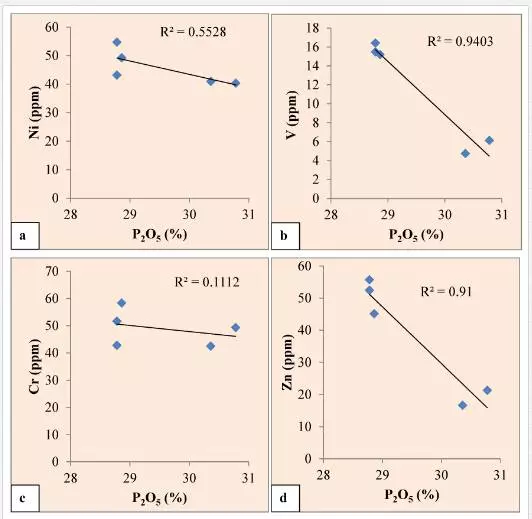
The lower concentration of copper, which may be meager evidence of pyrite, indicates highly oxidizing conditions of their formation. Due to the absence of sulfate (SO4) ions in the water the pyrite could not be precipitated at the time of phosphatization. The minor content of copper may be due to mutual ionic substitution of divalent Cu+2 by Fe+2 and Mg+2 during oxidizing conditions of the basin. The meager evidence of organic matter, near absence of sulfide minerals, and lower concentration of V, Ni, and Cu suggest that the phosphorites were deposited in an oxidizing environment slightly anaerobic to highly aerobic facies. In the present study, Ni, Cr, V, Zn show negative correlation with P2O5 (Fig. 6a,b,c,d) in all the samples of the area which may be due to mutual substitution within the apatite lattice that might have taken place during shallow marine environmental conditions of the basin.
The lower MgO content (0.18%) in the francolite of the phosphorites of the Duwi Formation compared to the Chile and Peru margins authigenic phosphorites proposed by [36]as 1.5% and 2.3%, respectively, can be attributed to the formation of the phosphatic grains in low MgO seawater [32]. The phosphorites of the study area also corroborate the findings of previous investigators showing low MgO concentrations. The same results were observed from Ogun phosphate rocks [33] and phosphorites of the Sonrai basin [6]. The study area shows good concentrations of P2O5. The P2O5 content of Matoon phosphorite deposits, district Udaipur (Rajasthan) is as high as 36% [37]. [38] reported that sediments of SE Coast of USA contain as high as 40% P2O5. The P2O5 content may be due to the presence of cryptocrystalline collophane as is commonly present in most Precambrian mudstones [39]. The silica content in Lalitpur phosphorites are in line with those of the Peru margin (8.79 wt%) [40] and Namibian margin phosphorites (19.4 wt%) [26]. The low concentration of TiO2 has also been reported from the Ogun phosphate rocks, Nigeria [33] which coincides to the TiO2 concentration of Lalitpur phosphorites indicating the presence of detrital heavy minerals like hematite, ilmenite, and rutile entrained in the sedimentary environment. This corroborates to a great extent the findings of [33]. The Lalitpur phosphorites show low concentration of MnO. The concentration of MnO in phosphorites has been reported by many earlier workers 0.38-0.4 wt% [41]; 0.014-0.021 wt% [42].
The concentration of Pb in phosphorites has been reported by many earlier investigators as10–43 ppm [43] and 3–4 ppm [44]. In the study area, uranium is an important trace element whose content in the phosphorite samples ranges from 1.67 to 129.67. The low concentration of U in few samples of the study area has also been noted in other phosphorite deposits of the world. The reason for low U content in phosphorites is due to its removal from the crystal lattice of carbonate-apatite during catagenesis [3]. The concentration of Zn in phosphorites has been reported by many earlier workers as 11–65 ppm [43]and 52–61 ppm [44]; [3] reported average concentration 614.51 ppm of phosphorites related to sedimentary rocks and 233.4 ppm related to endogenous rocks; others reported 18–33 ppm [45]; 104–130 ppm [35]; 30–290 ppm [46]; and 27.84–50 ppm [12].
Conclusion
The Paleoproterozoic metasedimentary rocks of Bijawar Group are overlain by the Archaean Bundelkhand Basement Complex and are underlain by the Vindhyan Supergroup. These were deposited as small intracratonic basins like Sonrai, Hirapur and Gwalior Basins. The lithostratographic units of the Bijawar Group in the Lalitpur district are better exposed and are divided into three formations: Berwar, Sonrai, and Solda Formations. Among these, phosphorites are found only in the Sonrai Formation.Megascopic study reveals that the brecciated phosphorite is reddish brown in color and fine to medium grained with angular fragments of chert and quartz embedded in a groundmass of iron oxides and secondary silica intercalated with minor veins of chert and iron oxides. The phosphorite horizon in the Lalitpur area is associated with pink to white brecciated massive quartzite, shale, dolomite and limestone of the basal unit. Thin-section photomicrographs showing pseudo-ooliths and pellets of collophane of phosphorites of the Lalitpur district.
The concentration trends of certain major oxides indicate that the phosphorites are more enriched in CaO, P2O5, and SiO2 than in Al2O3, Fe2O3, TiO2, Na2O, and K2O. The concentration trends of trace elements reveal that the phosphorites are moderately enriched in Co, Zn, Zr, Pb, and U than in Sc, Ba, V, Cr, Ni, Rb, Sr, Y, and Th. The dispersion pattern, correlation coefficient, and mutual relationship of significant major oxides, represented by plotted diagrams, indicate that SiO2, CaO, MgO are antipathetically related with P2O5. The relationship suggests a gradual replacement among these oxides during diagenesis. High values of P2O5 and CaO in the phosphorites indicate more concentration of apatite constituent. Poor correlation between MgO and P2O5 in phosphorites of the area indicates that Mg might not have been incorporated in the apatite structure. The low magnesium content in the phosphorites indicates that little dolomitization has occurred. The difference in geochemical behavior of CaO and MgO may be due to ionic substitution of Ca+2 by MgO+2 in the apatite crystal lattice during highly oxidizing, alkaline environment of the basin. The strong negative relationship between P2O5with Fe2O3 in phosphorites may be due to leaching and/or mild weathering of iron from the ores and reprecipitation along with P2O5 in the pore spaces, cavities/voids, veins, etc. in highly oxidizing marine environment of the Bijawar basin. The low concentration of alumina may be due to minor occurrences of clay minerals and major content of ferruginous minerals like hematite and ferric-oxides, etc. that constitute the cementing material of the phosphatic rocks. The presence of low Al2O3 in phosphorites indicates the humid to low temperature climatic conditions at the time of precipitation and phosphatization of these sediments in the area.
The lower concentration of copper which may be meager evidence of pyrite, indicates highly oxidizing conditions of their formation. The minimum evidence of organic matter, absence of sulfide minerals, and lower concentration of V, Ni, and Cu suggest that the phosphorites were deposited in an oxidizing environment slightly anaerobic to highly aerobic facies. In the present study, Ni, Cr, V, Zn show negative correlation with P2O5 in all the samples of the area, which may be due to mutual substitution within the apatite lattice that might have taken place during shallow marine environmental conditions of the basin.